
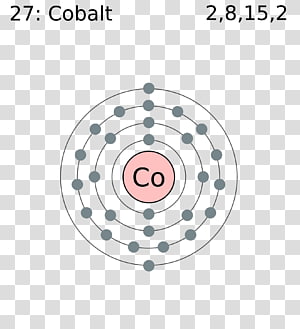
In a neutral atom, the number of protons is equal to the number of electrons in it. So those are the exceptions the main exceptions that you'll see when dealing with lifetime configuration.I am Sodium with Atomic Number \(11.\) How Many Electrons Do I Have?

So, again, I'm going to make this argon just make it for myself 4s1, 3d10 and again you might see it as argon 3d10, 4s1 same exact thing. That way this d orbital or d sublevel be completely filled which is very stable versus the s orbital will be halfway filled. Okay so anytime that it ends in d9 we're going to fix it up a little bit, same exact way we're going to take an electron from the 4s and we're going to move it over to the 3d. There are 2, 8, 15, 2 elements present in the 4 orbits of cobalt and can be represented in this form: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 7 4s 2.
Cobalt electron configuration full#
So let's make the electron configuration for that 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d9 from here on out anytime you see d9 we're going to change it. Full electron configuration can be defined as 27 electrons distribution in 4 shells of Co element. Electron Configuration and Oxidation States of Cobalt Electron configuration of Cobalt is Ar 3d7 4s2. So you might see either one but they're the same.ĭown here the other exception that you're going to see is copper, or anything along copper which we'll talk about. Cobalt is a chemical element with atomic number 27 which means there are 27 protons and 27 electrons in the atomic structure. So this is talking about the order of energy you might see it also looking like this, this is just doing it in order of number 3 comes before 4 but they're exactly the same thing, they're depicting the exact same thing, nothing different about them. You might see this is the diagram or the electron configuration that you're going to see and this is actually higher in energy than this. So instead we're going to write actually we're going to make this argon as we noted before in the noble gas configuration and we're going to make this 4s1, 3d5 this is halfway filled which is pretty stable and this is much more stable being half way filled rather than being the d4. Density is defined as the mass per unit volume. Typical densities of various substances are at atmospheric pressure. Density of Cobalt Density of Cobalt is 8.9g/cm3. So, it has partially filled 3d orbitals and presence of unpaired e s are.
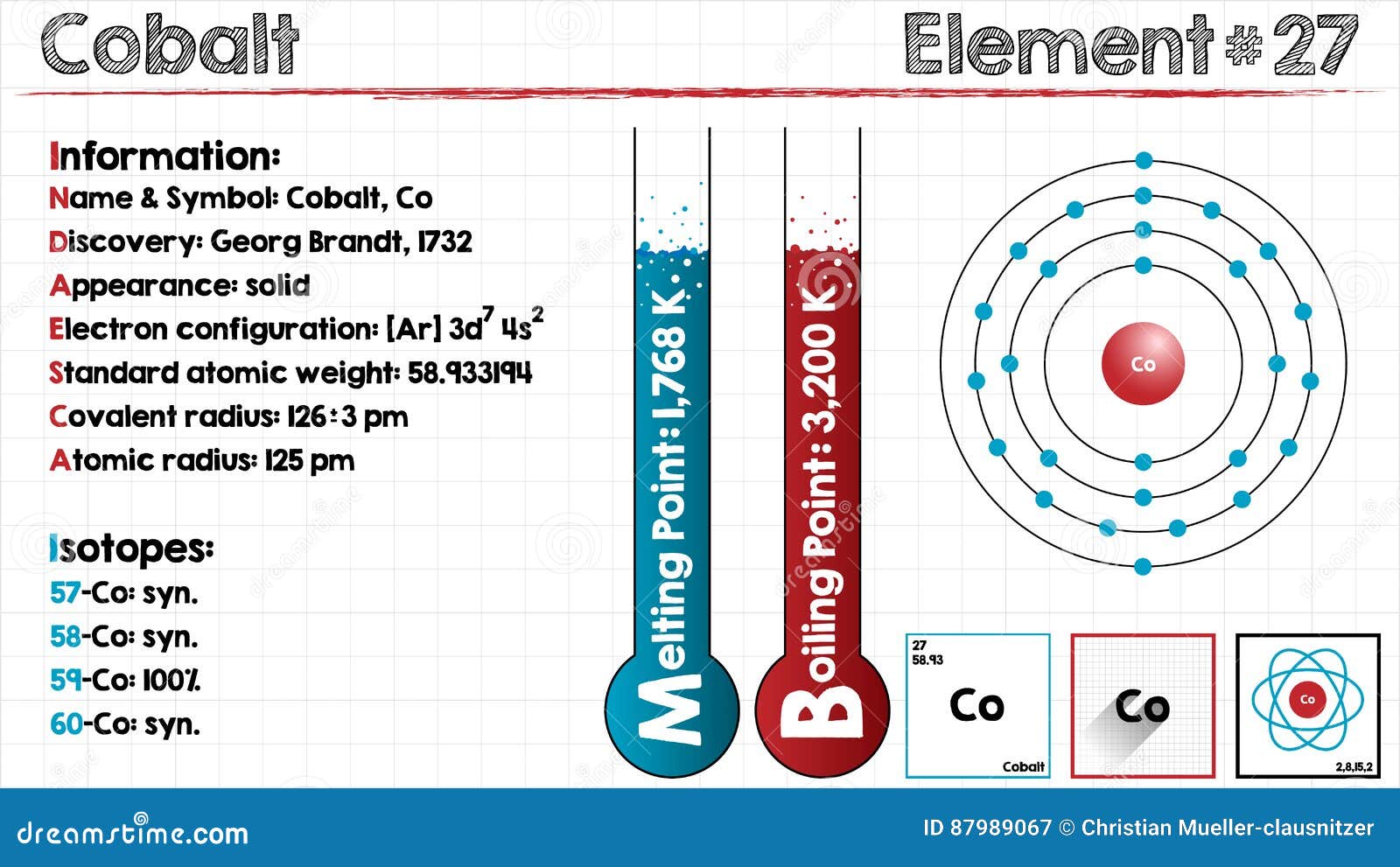
So what we're going to do, we're going to, that should be a 2 there, sorry, and we're going to take one of the electrons in the 4s orbital and move it over to the 3d orbital. Electron configuration of Cobalt is Ar 3d7 4s2. Cobalt(2+) Co+29 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety. its electronic configuration is AR 3d7 7 4s2 2. Right now we're 1 electron short of it being halfway filled. The d sublevel is more stable when its either half full all the orbitals are filled with at least 1 electron or completely filled. If we're going to make this short hand and make the electron configuration for this we would make this 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d4 okay from now on every time you see 3d4 you're going to change it, we do not like 3d4. This metal is intended to be alloyed with zinc and nickel. These are generally more expensive than nickel. Atomic Structure of Cobalt Atomic Radius: 1.67 Atomic Volume: 6.7cm3/mol Covalent Radius: 1.16 Cross Section (Thermal Neutron Capture)a/barns: 37.2. Basic Characteristics of Cobalt It is mainly used in high performance superalloys. Chromium is a transition metal and it has 24 electrons and here is the orbital diagram. The full electron configuration of cobalt is 1s2 2s2 2p6 3s2 3p6 3d7 4s2 while its abbreviated or simplified version is written as follows: Ar 3d7 4s2. Okay alright so let's talk about the exceptions you're going to see when you're dealing with electron configuration there's going to be a few around but we're going to talk about the main ones you're probably going to see in class. Terms in this set (25) What is the electron configuration of manganese 1s2 2s2 2p6 3s2 3p6 3d7 What is the electron configuration of cobalt 1s2 2s2 2p6 3s2.


 0 kommentar(er)
0 kommentar(er)
